Draw a valid Lewis dot structure for the following molecules a NCOb NF3 12 from CHEM 100 at Joliet Junior College. See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding.

Nf3 Lewis Structure And Molecular Geometry Youtube
Using a MO diagram determine.

. A Draw the Lewis structure for NF 3. Which of the following molecules have net dipole moments. See the answer See the answer done loading.
Part A NF3 Draw the molecule by placing atoms on the grid and connecting them with bonds. CH2O C2Cl4 CH3NH2 CFCl3 C central. Total electron pairs are determined by dividing the number total valence electrons by.
CF4 SeF4 KrF4 b. NF3 1 answer below. Click hereto get an answer to your question Draw the Lewis structures for the following molecules and ions.
Include all lone pairs of electrons. Count total valence electron in. Include all lone pairs of electrons and nonbonding electrons.
A C103- M 16. In each case the first atom listed is the central atom. OCl2 KrF2 BeH2 SO2 c.
There are two elements in NF 3. Draw a Lewis structure for each of the following molecules. Draw the Lewis dot structure for each of the following molecules or ions.
For the molecules that are polar indicate the polarity of each bond and the direction of. CH2O C2Cl4 CH3NH2 CFCl3 C central. Part B HBr Draw the molecule by placing atoms on the grid and connecting them with bonds.
Include all lone pairs of electrons. A step-by-step explanation of how to draw the NF3 Lewis Dot Structure Nitrogen trifluorideFor the NF3 structure use the periodic table to find the total n. NF3 HBr SBr2 CCl4 Write a Lewis structure for each molecule.
Lewis Structures Lewis Representation of Simple Molecules video tutorial 002906. Include all lone pairs of electrons and nonbonding electrons. Nitrogen in the NF3 Lewis structure with all three fluorine atoms arranged in a trigonal pyramidal geometry.
Textbook Solutions 12961 Important Solutions 9. B-cçs 10 c -s I 15. NF3 1 answer below.
Total valence electrons pairs. Draw the Lewis dot structure of the following. Lewis dot Structure for NF3 generated from step-1 and step-2.
NF3 HBr SBr2 CCl4 Write a Lewis structure for each molecule. Draw the Lewis structures for the following molecule and ion. POCl3 SO42 XeO4 PO43 ClO4.
1 Answer to Draw a Lewis structure for each of the following molecules. H2S SiCl4 BeF2 CO2 - 3 and HCOOH. Nf3 lewis structure is.
Include all lone pairs of electrons and nonbonding electrons. The Lewis dot structure for any molecule can be found by following a general set of rules consisting of 5 or sometimes 6 steps. See Exercises 89 and 90 a.
Up to 24 cash back Lewis Structures VSEPR Polarity IM Forces. The NF3 is polar because NF3 has a lewis structure and the molecules form in a pyramidal. Draw the Lewis Dot Structure for the Hydrogen atom.
Add valence electrons around the fluorine atom as given in the figure. Include all lone pairs of electrons. Draw a Lewis structure for each of the following molecules.
Nitrogen in the NF3 Lewis structure with all three fluorine atoms arranged in a trigonal pyramidal geometry. Indicate the geometric shape and polarity of the following molecules. Draw a Lewis structure for each of the following molecules.
Include all lone pairs of electrons and nonbonding electrons. Draw the Lewis structures for the following molecules and ions H2S SiCl4 BeF2 CO32- HCOOH asked Dec 22 2020 in Chemical Bonding by Aashi01. SO3 NF3 IF3 d.
Draw three possible resonance structures for OCS. According to Lewis dot structure the number of bond around central atom is greater than four for which of the following anion. NF3 HBr SBr2 CCl4.
Steps of drawing lewis structure of NF 3 Total number of electrons of the valance shells of NF 3. This type of Lewis dot structure is represented by an atomic symbol and a series of dots. Arrow_forward Write total number of valence electrons draw Lewis Structures of these three compounds AND justify their 3D.
Write a Lewis structure for each molecule. Include all lone pairs of electrons. Draw a Lewis structure for each of the following molecules.
NF3 has a tetrahedral geometric structure and a trigonal pyramidal shape one nonbonding electron pair on Nitrogen. Draw a Lewis structure that obeys the octet rule for each of Draw a Lewis structure that obeys the octet rule for each of the following molecules and ions. Answer to Draw Lewis structures and predict the molecular structures of the following.
Chemistry questions and answers. You must draw diagrams for each molecule. Since Hydrogen is in Group I it has one 1 valence electron in its shell.
CBSE CBSE Science Class 11. Indicate and briefly explain which structure is the most important. View solution Which of the following structure is the most preferred structure for S O 3.
CH2O C2Cl4 CH3NH2 CFCl3 C central. Determine the number of bonding and nonbonding electron domains and indicate their electron domain and molecular geometries. Connect the exterior and core central atom of the NF3 molecule with three single N-F bonds.
A step-by-step explanation of how to draw the NF3 Lewis Dot Structure Nitrogen trifluorideFor the NF3 structure use the periodic table to find the total n. Using BEST Lewis structures and the VSEPR model match each molecular formula with the molecular geometry that describes it. 8 rows Simple steps for drawing the NF3 lewis dot structure.
There are two elements in NF 3.
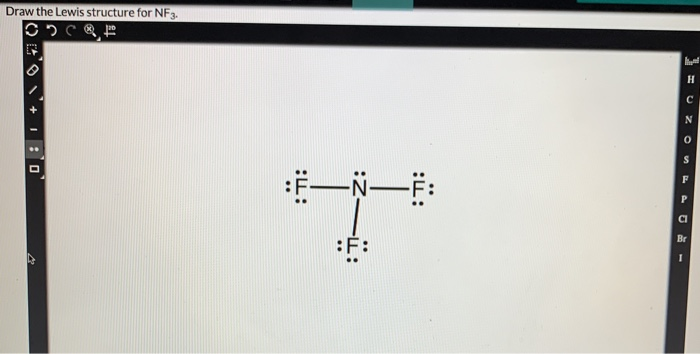
Solved Draw The Lewis Structure For Nf3 Part 2 1 Point Chegg Com

Nf3 Lewis Structure Nitrogen Trifluoride Youtube

Nf3 Lewis Structure Molecular Geometry Bond Angle Polarity Electrons
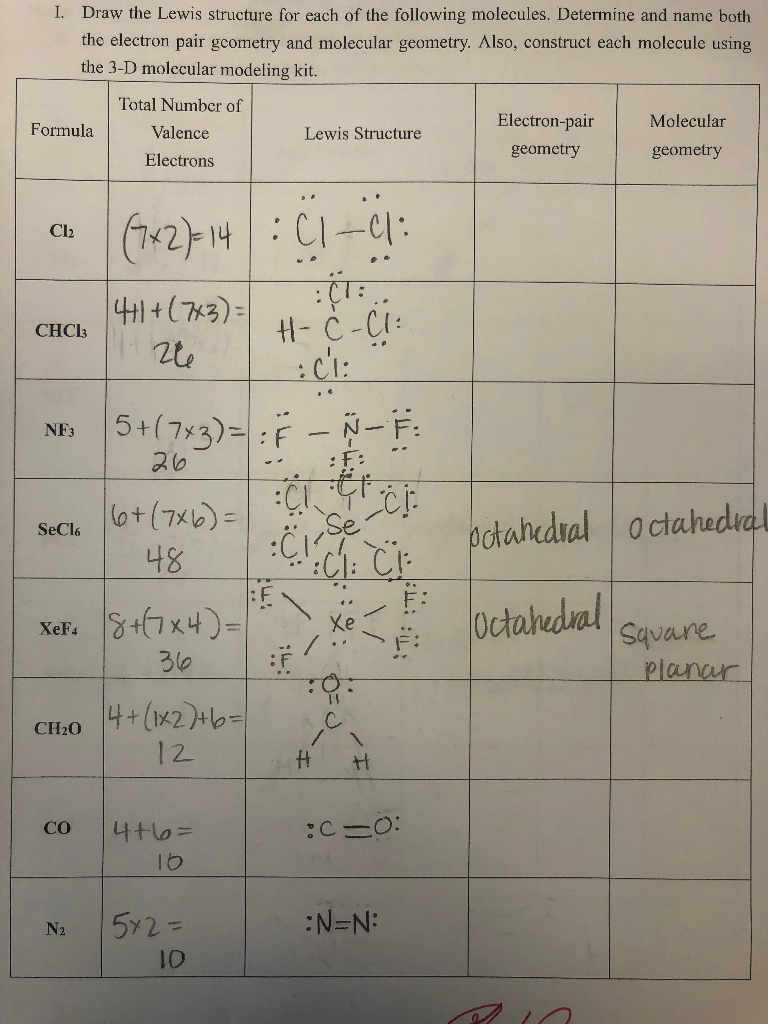
Solved Draw The Lewis Structure For Each Of The Following Chegg Com
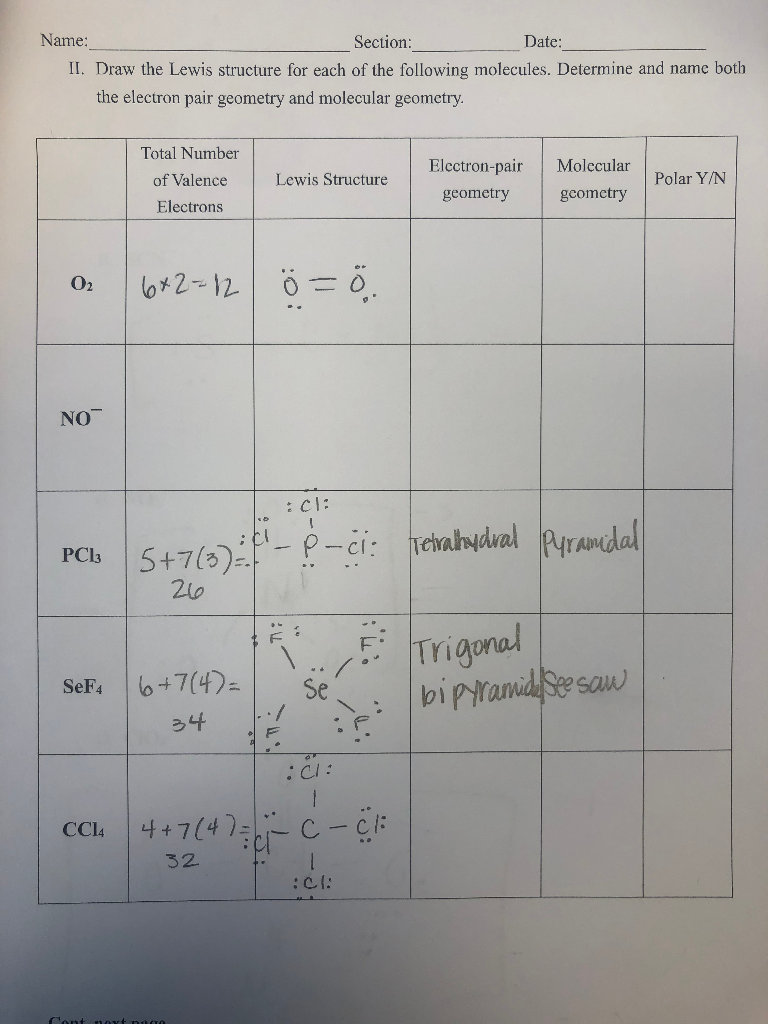
Solved Draw The Lewis Structure For Each Of The Following Chegg Com
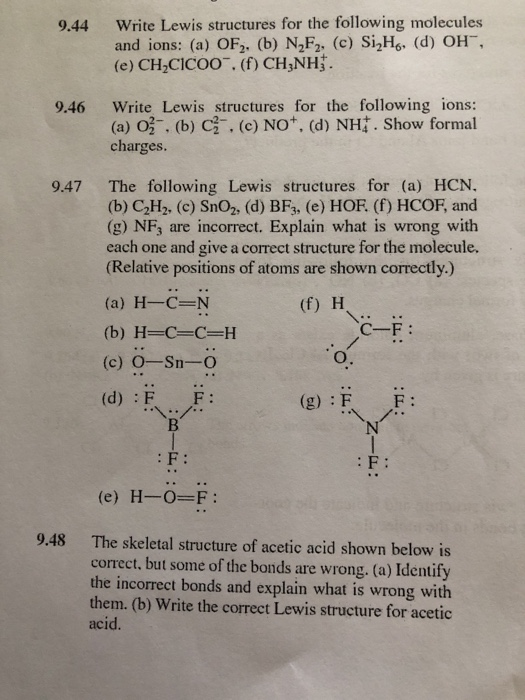
Solved Write Lewis Structures For The Following Molecules Chegg Com

Draw The Lewis Structures For The Following Molecules And Ions H2s Sicl4 Bef2 Co 2 3 And Hcooh
0 comments
Post a Comment